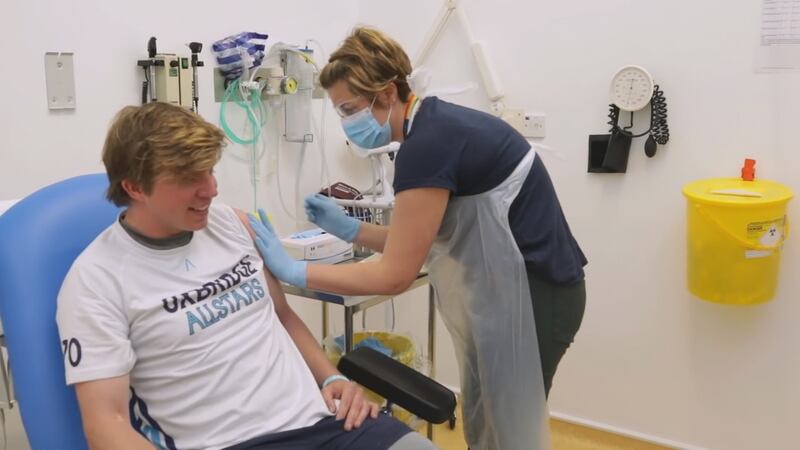
ATLANTA — Health officials are busy working to develop a vaccine for COVID-19, and one of the most important things they need during the late-stage trials are diverse volunteers.
It’s the thought process 32-year-old Atlanta resident Ashley Nealy applied when she decided to answer a social media ad looking for volunteers to take part in a coronavirus vaccine clinical trial. Nealy, an African-American said she felt it was important to make sure the trials were as diverse as possible.
Nealy told Channel 2 anchor Jovita Moore, she never hesitated to sign up.
“I actually wanted to do a clinical trial before I even saw the ad I had registered at the website that Dr. Fauci mentioned, the COVID prevention network, and I never heard a call back. So whenever I saw the ad, I was like, let me just see if they’ll accept me for this trial. So I signed up and less than two hours later actually got a call to participate in the trial,” Nealy said.
“So I think everyone’s trying to figure out how they can help stop this pandemic. And this was one of the ways I figured I could help give back. I know, they said that there cannot be a vaccine produced without more African Americans taking place in the trial. So I figured that I can put my name in a hat and see if I can help be part of that,” Nealy added.
TRENDING STORIES:
- UGA reports 800+ COVID-19 cases on campus, expert says real number could be more than 3 times that
- Joe Biden speaks exclusively with Channel 2 on election in Georgia, COVID-19 response
- Woman’s routine procedure lands her with massive unexpected bill -- why a new law may not help her
Moore asked Nealy why she was willing to do participate in the trial knowing it could impact her health and take up a lot of her time.
“This is so that I can help stop the pandemic and make sure that the vaccine works for Black Americans and everyone whenever it comes out,” Nealy said.
“You know, so many people will say that they sort of have a mistrust. You’re like a guinea pig, you don’t know what’s going to happened. You don’t know what you’re being injected with. What would you say to those people who have a real fear or maybe just a lack of interest right now?” Moore asked.
“Yeah, I will say I definitely understand. I know Black people in particular have a really long mistrust history with public health and with us being experimented on. And I understand that 100%. I will say if you are willing, and maybe if you’re like a guinea pig like me to definitely participate, because we really can’t move forward on this pandemic without knowing that a vaccine works for all of us. I think I found some comfort in knowing that the pandemic coming that the trial was a double blind study, so there was a chance I may not get the vaccine, I can get a placebo so that actually helped me a lot to make up my mind,” Nealy responded.
The 32-year-old went into the trial knowing she could end up with side effects. She says so far, they’ve not been an issue.
“So actually, the next day after getting the vaccine, I did feel tired. I wasn’t expecting to feel that fatigue. And I did have some body aches and sweating. And that was some of the things that they said you might experience if you have the vaccine. And then of course, they can’t tell us but I’m pretty sure I did. And those are only symptoms I had. They only lasted about a day and the next day I was fine,” said Nealy.
Cox Media Group