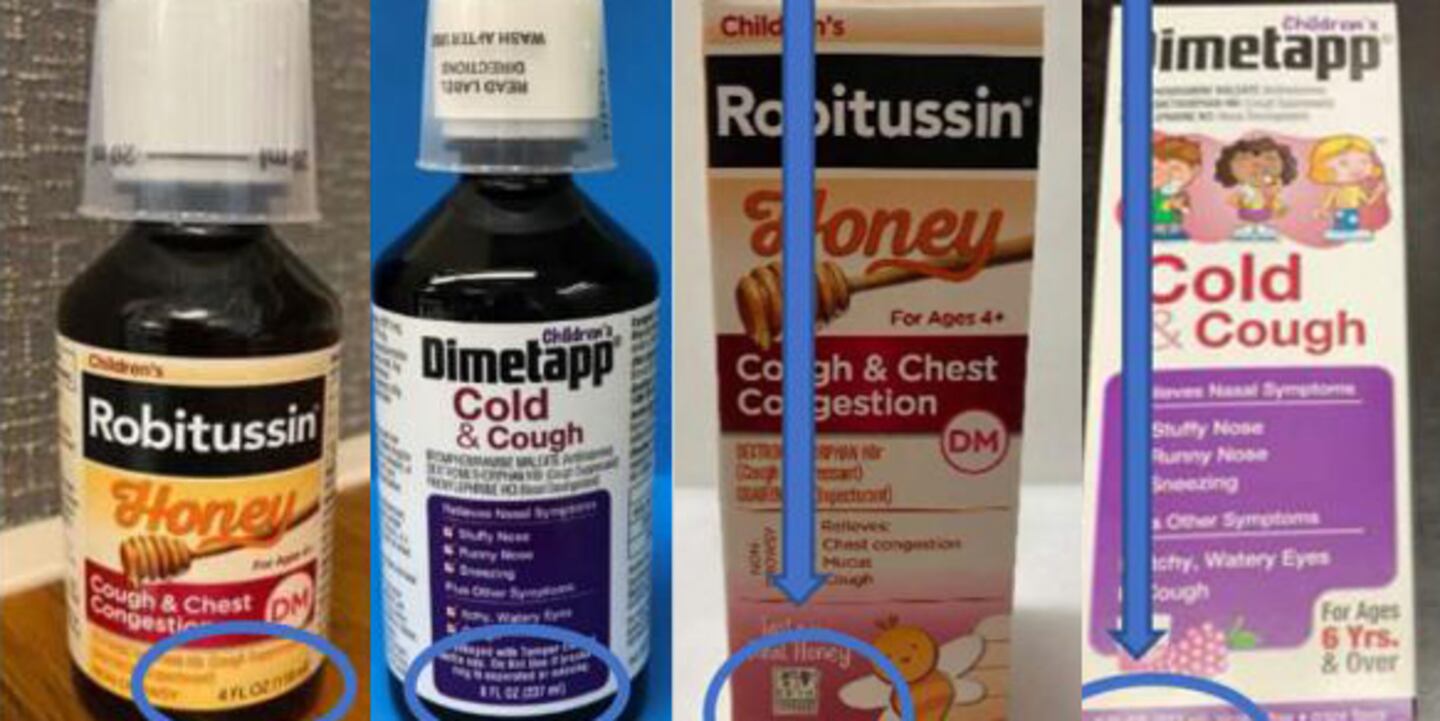
GSK Consumer Healthcare, the joint venture between GlaxoSmithKline and Pfizer, issued a voluntary recall of some of its children’s Robitussin and Dimetapp after it was discovered that incorrect dosing cups were included on some products.
According to the recall some cups were missing both 5 milliliter and 10 milliliter marks raising concern that overdosing could occur. The cups packaged with both recalled products only had the 20 milliliter mark.
The FDA posted the company announcement on it’s website which included symptoms of an overdose:
There is a potential risk of accidental overdose if caregivers dispensing the syrup do not notice the discrepancies between the graduations printed on the dosing cups and the indicated amounts to be administered (as directed in the instructions for use). Children’s Robitussin Honey Cough & Chest Congestion DM contains 10 mg dextromethorphan HBr USP and guaifenesin USP 100 mg per 10 mL, and is labeled for children 4 and older, as well as adults. Children’s Dimetapp Cold & Cough contains 2 mg brompheniramine maleate USP, 10 mg dextromethorphan HBr USP, and 5 mg phenylephrine HCl USP per 10 mL, and is labeled for children 6 and older, as well as adults. Symptoms of overdose of either product may include any of the following: impaired coordination; brain stimulation causing increase in energy, elevation in blood pressure, heart rate, and respiration; a lack of energy and enthusiasm; severe dizziness or drowsiness; slow heart rate; fainting; psychotic behaviour; restlessness; seizure; decreased respiration; nausea; vomiting; constipation; diarrhea; abdominal pain; visual and hearing hallucinations; urinary retention. As of the date of the recall announcement, GSK Consumer Healthcare has not received any adverse events related to these products or consumer complaints regarding the incorrect dosing cups supplied with the product.
The products were distributed nationwide between February 5, 2020 and June 3, 2020 and the recall is limited to the three lots listed below:
| Children’s Robitussin® Honey Cough and Chest Congestion DM (4oz) NDC 0031-8760-12 Lot: 02177 (Exp. Jan. 2022) Lot: 02178 (Exp. Jan. 2022) | Children’s Dimetapp® Cold and Cough (8oz) NDC 0031-2234-19 Lot: CL8292 (Exp. Sep. 2021) |
To see more on the company announcement on the FDA website click here.
Cox Media Group